A multicenter, randomized, controlled clinical trial evaluting the use of dehydrated human amnion and multilayer compression therapy in treatment of VLU
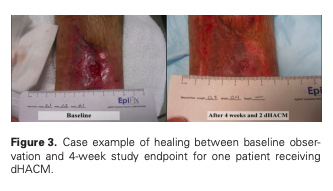
ABSTRACT: Venous leg ulcers produce significant clinical and economic burdens on society and often require advanced wound therapy. The purpose of this multicenter, randomized, controlled study is to evaluate the safety and efficacy of one or two applications of dehydrated human amnion/chorion membrane allograft and multilayer compression therapy vs. multilayer compression therapy alone in the treatment of venous leg ulcers. The primary study outcome was the proportion of patients achieving 40% wound closure at 4 weeks. Of the 84 participants enrolled, 53 were randomized to receive allograft and 31 were randomized to the control group of multilayer compression therapy alone. At 4 weeks, 62% in the allograft group and 32% in the control group showed a greater than 40% wound closure (p = 0.005), thus showing a significant difference between the allograft-treated groups and the multilayer compression therapy alone group at the 4-week surrogate endpoint. After 4 weeks, wounds treated with allograft had reduced in size a mean of 48.1% compared with 19.0% for controls. Venous leg ulcers treated with allograft had a significant improvement in healing at 4 weeks compared with multilayer compression therapy alone.
A-multicenter-randomized-controlled-clinical-trial-evaluting-the-use-of-dehydrated-human-amnion-and-multilayer-compression-therapy-in-treatment-of-VLU