An aseptically processed, acellular, reticular, allogenic human dermis follow-up study
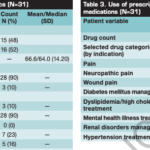
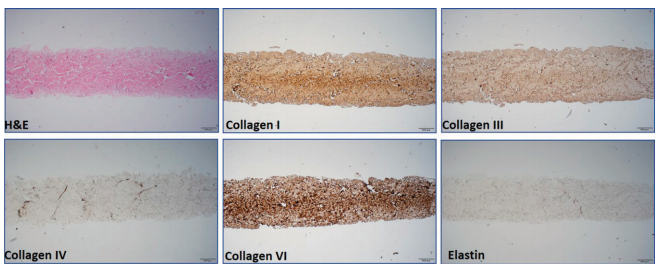
Abstract: Aseptically processed human reticular acellular dermal matrix (HR-ADM) has been previously shown to improve wound closure in 40 diabetic patients with non-healing foot ulcers. The study was extended to 40 additional patients (80 in total) to validate and extend the original findings. The entire cohort of 80 patients underwent appropriate offloading and standard of care (SOC) during a 2-week screening period and, after meeting eligibility criteria, were randomised to receive weekly applications of HR-ADM plus SOC or SOC alone for up to 12 weeks. The primary outcome was the proportion of wounds closed at 6 weeks. Sixty-eight percent (27/40) in the HR-ADM group were completely healed at 6 weeks compared with 15% (6/40) in the SOC group. The proportions of wounds healed at 12 weeks were 80% (34/40) and 30% (12/40), respectively. The mean time to heal within 12 weeks was 38 days for the HR-ADM group and 72 days for the SOC group. There was no incidence of increased adverse or serious adverse events between groups or any graft-related adverse events. The mean and median HRADM product costs at 12 weeks were $1200 and $680, respectively. HR-ADM is clinically superior to SOC, is cost effective relative to other comparable treatment modalities, and is an efficacious treatment for chronic non-healing diabetic foot ulcers
An-aseptically-processed-acellular-reticular-allogenic-human-dermis-follow-up-study