Effectiveness of Dehydrated Human Amnion for Treatment of Lower Extremity Diabetic Ulcers
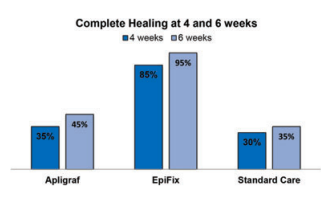
Abstract: A prospective, randomised, controlled, parallel group, multi-centre clinical trial was conducted at three sites to compare the healing effectiveness of treatment of chronic lower extremity diabetic ulcers with either weekly applications of Apligraf® (Organogenesis, Inc., Canton, MA), EpiFix® (MiMedx Group, Inc., Marietta, GA), or standard wound care with collagen-alginate dressing. The primary study outcome was the percent change in complete wound healing after 4 and 6 weeks of treatment. Secondary outcomes included percent change in wound area per week, velocity of wound closure and a calculation of the amount and cost of Apligraf or EpiFix used. A total of 65 subjects entered the 2-week run-in period and 60 were randomised (20 per group). The proportion of patients in the EpiFix group achieving complete wound closure within 4 and 6 weeks was 85% and 95%, significantly higher (all adjusted P-values≤0⋅003) than for patients receiving Apligraf (35% and 45%), or standard care (30% and 35%). After 1 week, wounds treated with EpiFix had reduced in area by 83⋅5% compared with 53⋅1% for wounds treated with Apligraf. Median time to healing was significantly faster (all adjusted P-values≤0⋅001) with EpiFix (13 days) compared to Apligraf (49 days) or standard care (49 days). The mean number of grafts used and the graft cost per patient were lower in the EpiFix group campared to the Apligraf group, at 2⋅15 grafts at a cost of $1669 versus 6⋅2 grafts at a cost of $9216, respectively. The results of this study demonstrate the clinical and resource utilisation superiority of EpiFix compared to Apligraf or standard of care, for the treatment of diabetic ulcers of the lower extremities.