Safety and Efficacy of an Autologous Blood
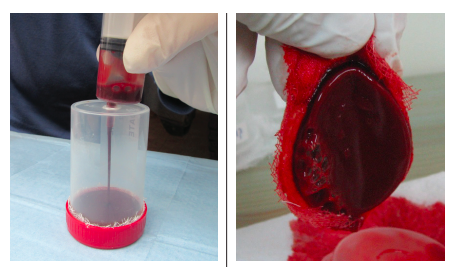
Objective: This pilot study evaluates safety in terms of the occurrence of adverse events (AEs) as well as the efficacy in terms of complete wound healing rates of a blood clot product when applied to chronic neuropathic diabetic foot ulcers (DFUs). Materials and Methods. Participants were chosen from patients with DFUs visiting the wound care clinic. Up to 10 mL of blood drawn from each participant was injected into the product’s clotting tray. Within 12 minutes, the blood clot product was formed, applied to the single DFU of each participant, and covered with primary and secondary dressings. Patients received up to 12 blood clot product applications every 5 to 9 days for up to 12 weeks.
Safety-and-Efficacy-of-an-Autologous-Blood